
The Earth’s atmosphere is a delicate blanket of gases that envelops our planet, providing the necessary conditions for life to thrive. Comprising various gases in different proportions, this atmosphere plays a crucial role in regulating temperature, protecting life from harmful radiation, and sustaining vital processes like photosynthesis. Understanding its composition and the methods for separating its primary constituents, nitrogen and oxygen, is essential for various industrial, scientific, and environmental endeavors.
Composition of Earth’s Atmosphere:
Earth’s atmosphere is primarily composed of nitrogen (N2), oxygen (O2), argon (Ar), and traces of other gases such as carbon dioxide (CO2), neon (Ne), helium (He), methane (CH4), and ozone (O3). The most abundant gases are nitrogen and oxygen, which together constitute approximately 99% of the atmosphere’s total volume.
- Nitrogen (N2): Nitrogen makes up about 78% of the Earth’s atmosphere. It is crucial for various biological processes and is used extensively in industrial applications, such as in the production of ammonia for fertilizers and in the manufacturing of electronics.
- Oxygen (O2): Oxygen accounts for roughly 21% of the atmosphere. It is vital for respiration in most organisms and is also widely utilized in industrial processes, including metal smelting, combustion, and medical applications.
- Argon (Ar): Argon constitutes around 0.9% of the atmosphere and is primarily used in welding and as an inert gas in various industrial processes.
Methods of Separating Nitrogen and Oxygen:
Fractional Distillation:

LIQUID NITROGEN/OXYGEN STORAGE EQUIPMENT FOR INDUSTRIAL USE
Fractional distillation is based on the principle of differences in boiling points of gases. Air is first cooled and compressed to liquify it. Then, the liquid air is allowed to boil slowly. As the temperature increases, gases with lower boiling points, such as nitrogen and oxygen, vaporize first. By collecting these vapors separately and condensing them, nitrogen and oxygen can be obtained in their pure forms. Fractional distillation is a well-established and efficient method for large-scale production of nitrogen and oxygen. Fractional distillation requires significant energy input due to the need for cooling and compressing air. Additionally, it is a capital-intensive process, making it less suitable for small-scale applications.
Membrane Separation:
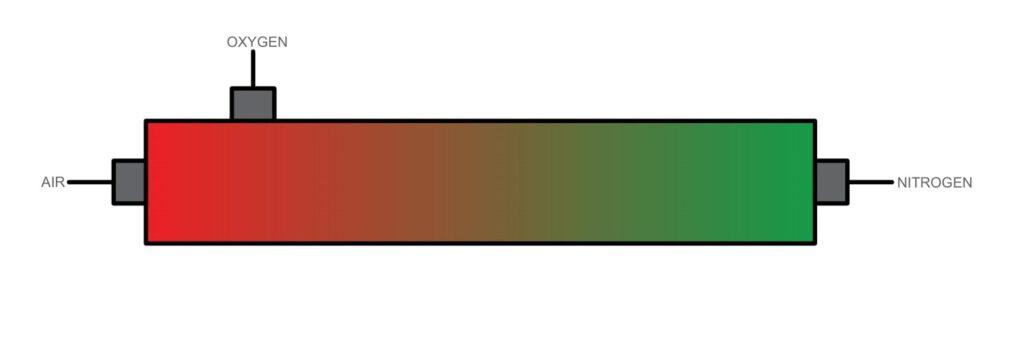
Membrane separation relies on permeation through selective membranes. In this method, air is passed through a membrane that selectively allows certain gases, such as nitrogen, to permeate more readily than others, such as oxygen. By controlling factors like pressure, temperature and membrane material, the desired gases can be separated. Membrane separation is energy-efficient at lower purities and scalable. It can be employed for both large-scale industrial processes and smaller applications. It also offers flexibility in terms of operation and requires less maintenance compared to other methods.
Pressure Swing Adsorption (PSA):
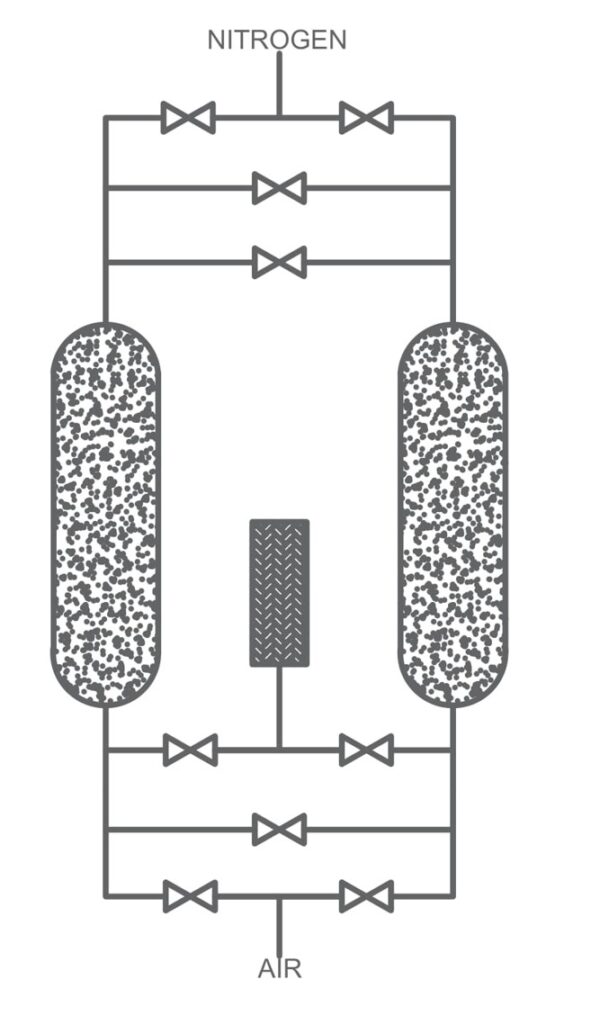
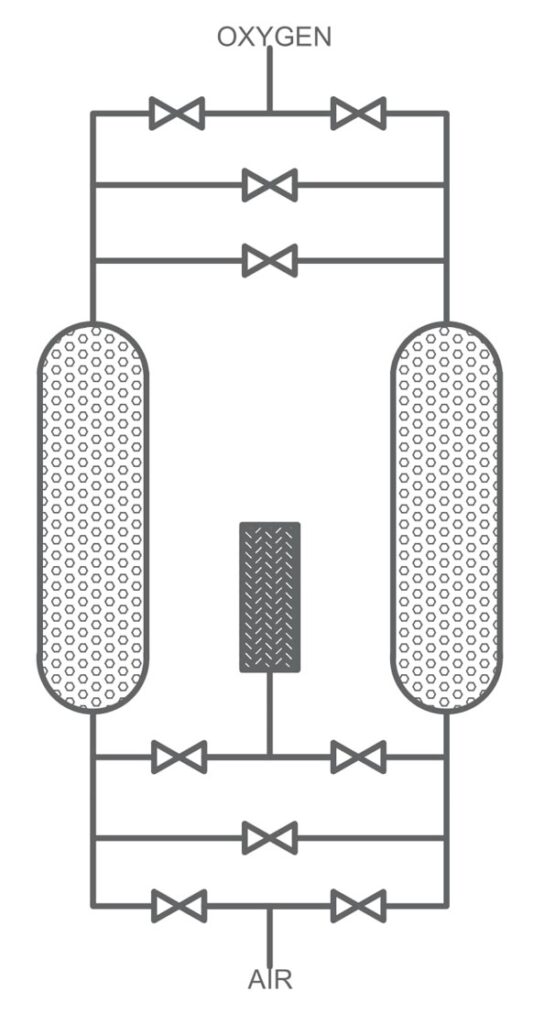
PSA involves passing air through a bed of adsorbent material, such as carbon molecular or zeolite sieve, at high pressure. The adsorbent selectively adsorbs oxygen or nitrogen while allowing other gas to pass through. After a period, the pressure is reduced, allowing the adsorbed oxygen or nitrogen to desorb and be released. PSA is highly efficient and can produce gases with high purity levels. It is the most suitable technology for on-site gas generation of nitrogen and oxygen and can be automated for 24/7 operation.
The composition of Earth’s atmosphere, dominated by nitrogen and oxygen, is essential for sustaining life and supporting various industrial processes. Understanding the methods for separating these gases is crucial for applications ranging from medical oxygen supply to industrial gas production. While each separation method has its advantages and limitations, advancements in technology continue to refine these processes, making them more efficient, cost-effective, and environmentally friendly. As we delve deeper into the intricacies of atmospheric science and gas separation techniques, we unlock new possibilities for innovation and sustainable development.
Enquire Now

